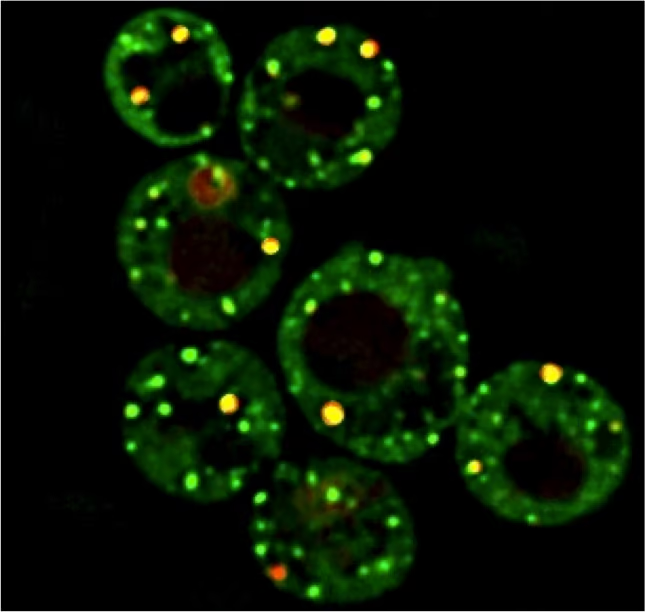
Stress granules and P-bodies are cytoplasmic biomolecular condensates conserved throughout eukaryotes. They consist of non-translating mRNAs, RNA-binding proteins, and many additional proteins, RNAs and small molecules. They are induced under conditions of cellular stress, where they may facilitate cell survival as part of the integrated stress response. Dysregulation of stress granule assembly and clearance in particular is linked to the pathology of various cancers and neurodegenerative diseases, including ALS.
Though much is known about stress granule and P-body assembly mechanisms and composition, their underlying functions and how cells clear them remain poorly understood. These topics are of key interest to our lab.
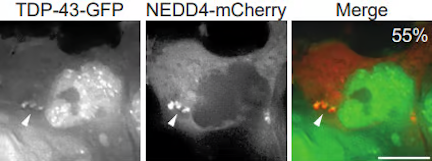
Amyotrophic Lateral Sclerosis (ALS) is a fatal disease driven by motor-neuron atrophy, with no effective treatment or cure at present. Like many neurodegenerative diseases, defects in proteostasis occur; in particular, cytoplasmic mislocalization, accumulation and aggregation of an RNA-binding protein called TDP-43 is strongly implicated in ALS pathology for the vast majority of patients.
TDP-43 can localize in stress granules, and during our investigations of stress granule clearance mechanisms, we discovered a surprising new means of cytoplasmic TDP-43 degradation involving TDP-43 targeting to the endolysosomal pathway. We continue to work in this area to better understand mechanistically how TDP-43 degradation, including of so-called “toxic” species, can be upregulated, which may identify novel therapeutic targets in ALS.
More broadly, we are interested in what other cytoplasmic proteins degrade via this endolysosomal mechanism, and what governs the use of different degradation mechanisms for a given protein substrate.
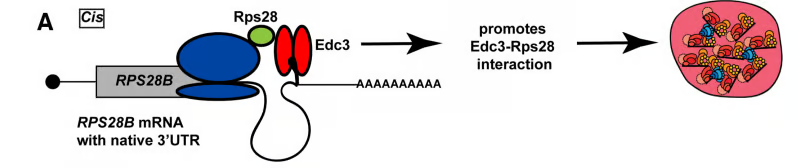
How protein-protein interactions are correctly and efficiently established is not straightforward given the complexity of the cellular milieu, off target interactions, barriers to diffusion and other factors. Spatiotemporally regulated mechanisms for facilitating the co-translational establishment of protein interactions have been described, but how widespread such mechanisms are is unclear. Recently, we described the first example in yeast of an mRNA’s 3’UTR playing a role in aiding interaction of a nascently translated protein (Rps28b) with a binding partner (Edc3), which in turn helps drive the formation of P-bodies. These findings mimic similar observations for different mRNAs and proteins by the Mayr lab in mammalian cells (see additional links below).
We are interested in determining how prevalent mRNA 3’UTR-based scaffolding of nascent protein interactions is and understanding the key mechanisms underlying this process.
Additional links:
https://www.mskcc.org/research/ski/labs/christine-mayr/overview
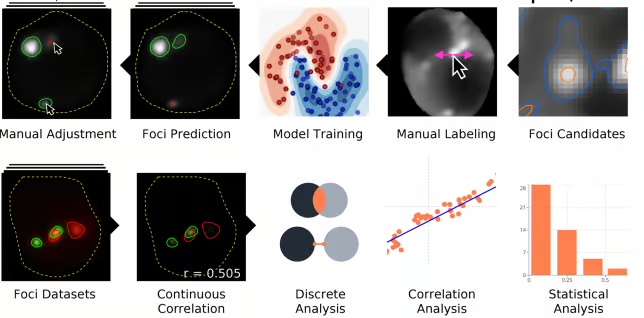
A significant problem in quantifying microscopic foci such as stress granules and P-bodies is the unambiguous identification of what is a “real” foci, versus something that is artifactual. Additionally, the intensity of a stress granule foci relative to the background can also be variable, including between cells; this is a frequent problem in yeast live cell microscopy.
To combat these issues, Ilya Shabanov developed a freely available tool called HARLEY which runs in the chrome browser, and which allows intuitive user-based model training to reproducibly and automatically identify foci of interest in yeast. This is particularly important as user reproducibility in foci quantification is far from perfect, as we discovered based on testing multiple users reproducibility in scoring stress granules. Numerous other tools relating to foci quantification, including extensive co-localization metrics, are also part of the HARLEY package.
We hope to expand HARLEY’s applicability to other biological models in the future.
Additional links: